The EQUATOR Network and Research Reporting Guidelines: What does it mean for authors?
Sept 2015Across the more than 30,000 biomedical journals being published, how can editors assess the quality of your article over another? How can readers be assured that valuable information about research methods hasn’t been omitted, or that the outcomes have not been selectively reported? Errors such as these can lead to inaccurate conclusions, which in turn can have a serious impact on medical practice and decision making. In order to assist authors, reviewers and editors, and provide effective quality control across all biomedical journals, the EQUATOR Network (Enhancing the QUAlity and Transparency Of health Research) “develops and maintains a comprehensive collection of online resources providing up-to-date information, tools, and other materials related to health research reporting.”
EQUATOR Network
Founded in 2006, the EQUATOR Network publishes over 250 research reporting guidelines and operates 3 centers in the United Kingdom, Canada, and France that raise awareness and support adoption of good research reporting practices. The EQUATOR Network website (www.equator-network.org) maintains a comprehensive, up-to-date list of guidelines and a series of toolkits designed for authors, editors, developers, librarians, and teachers.
Research Reporting Guidelines
Guidelines provide specific recommendations for the reporting of different types of research. They are written by experts in study design, epidemiology, biostatistics, and research methodology, and have been developed to accommodate both general research types (e.g., randomized trials, systematic reviews) and more specific ones (e.g., reporting body fluid biomarker research studies in neurologic disorders). Many of these guidelines include detailed checklists of items that can be included in your manuscript as part of a submission, and in some cases a flow diagram that displays the progress of all participants through the trial is also included (see Table 1).
Table 1. The most commonly used reporting guidelines
| Study Type | Guidelines | Items |
|---|---|---|
| Randomized trials | CONSORT | Checklist and flow diagram |
| Observational studies | STROBE | Checklist |
| Systematic reviews | PRISMA | Checklist and flow diagram |
| Case reports | CARE | Checklist |
| Quality improvement studies | SQUIRE | Checklist |
| Diagnostic accuracy studies | STARD | Checklist and flow diagram |
| Economic evaluation studies | CHEERS | Checklist |
Checklists and Flow Diagrams
Increasingly, many journals require the inclusion of completed guideline checklists as part of the submission process. In 2011, Neurosurgery began requiring the submission of completed checklists for systematic reviews, randomized trials, and observational studies. In 2014, Medicine restructured its article types to match the eight main research types and required that all submissions be submitted in compliance with the corresponding research reporting guideline.
Of note, checklist items are not intended to increase the work for authors but to confirm that the essential elements of the manuscript are present. For example, the CARE checklist for the writing of case reports requires that “The words ‘case report’ should be in the title along with the area of focus” (see Figure 1). The STROBE checklist for the reporting of observational studies outlines that in the Discussion section of your manuscript, the author must “Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias.”
Figure 1. Example of a Guideline Checklist
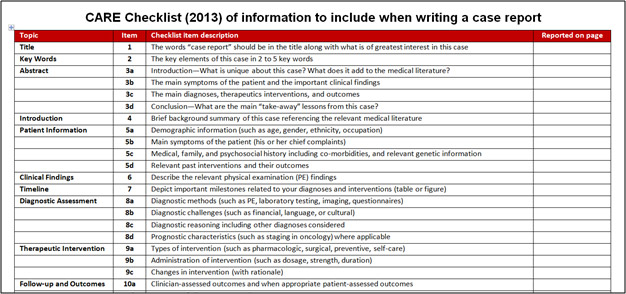
Checklists and flow diagrams are generally available for download as either Word or PDF files. Each individual title may include in the Instructions for Authors specific directions on how to provide evidence of compliance, but in most cases checklists are to be included as supplemental submission items. The best approach would be to contact the editorial office of the journal you are submitting your article to and confirm their policies regarding reporting guidelines. Even when not required, guidelines can still provide a helpful foundation for both composing and revising your work.
Advantages of Compliance
Compliance with these guidelines, as indicated by including the completed checklist (and flow diagram, if applicable) with your submission, provides editorial offices with an indicator of the thoroughness of your submission. However, there are additional advantages that compliance provides your paper submission. The work of reviewers and editors when assessing your submission is made far easier when they are provided with a consistent and readily recognizable submission structure, which could help speed up the time necessary for you to receive a decision. You should not view the guidelines as an imposition requiring further effort, but as an indispensable author tool in the improvement of your submission. As journals have adopted guidelines to bring themselves in line with the top-tier of biomedical journals, authors can likewise present themselves as producing the highest quality submissions.
Authors can use the author toolkit on the Equator Network site to understand how to incorporate guidelines and best practice into their writing: http://www.equator-network.org/toolkits/authors/#auwrit.
 Back to Author Resource Review
Back to Author Resource Review